Soil Test? Why do I need one of those? Why is it important? What are you looking for in a soil test, anyway? 🧐

A quality soil test is the best starting point for any lawn fertilization program. Here at GreenGate, putting together a comprehensive lawn care plan starts on day one with a quality soil sample and analysis. By providing a free soil sample to every new customer signing up with our 8-step lawn care program or one of our lawn bundles, we can fully understand what’s going on with your soil’s chemical makeup. With this information, we can design a plan unique to your yard, helping to maximize your lawn care investment.
Great lawn care starts with having an understanding of the soil composition. For example, a high pH level is a death sentence for thick, lush green grass because it compacts and locks up the soil, rendering the turf unable to access the nutrients provided to it (no matter how good the lawn fertilizer program is). Compare it to having a really yummy milkshake, but the only way to enjoy it is through a tiny martini straw!
This article will review some of the terms seen in a soil analysis report. We hope to educate you on their meaning, why they are important, and suggestions for improvement. We will not cover Trace Elements in this review.
Soil Composition
Total Exchange Capacity: Optimum is 12
Joe, with Turf Care Supply, puts it best. He states that strictly by definition, CEC measures how many cations can be retained on soil particle surfaces. In other words, some soils are good at holding water and nutrients in place, while others are poor. To understand how CEC works, we need to go back and review some basic high school chemistry concepts.
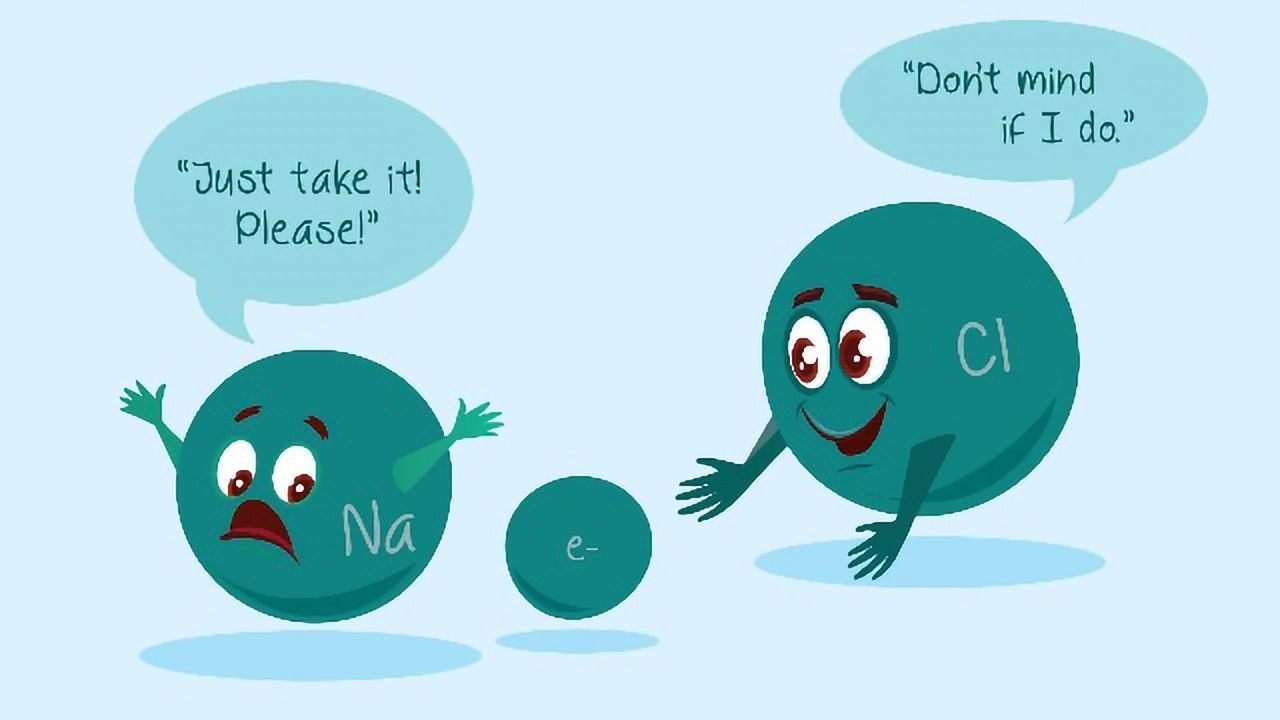
In nature, many elements and ions have either a positive or negative charge. Just as with electromagnets, opposites attract. Take, for example, table salt (sodium chloride or NaCl). In nature, a sodium (Na) atom has a positive charge of +1, and a chlorine (Cl) atom has a negative charge of -1. When the two elements interact, they form an ionic bond, and the two charges neutralize each other, meaning the net charge of NaCl is 0. This is important because most macro and micronutrient plants’ needs aren’t delivered in their pure elemental form; they are delivered in ion form and are derived from ionic compounds.
For example, plants need potassium (K), but we don’t spread pure potassium on the ground because it is highly reactive in its elemental form. Instead, we spread muriate of potash (MOP) which has a chemical formula of KCl (otherwise written K+ Cl-). Much like table salt dissolves in water into Na+ and Cl- ions, when MOP is spread onto the turf, water eventually splits up the K+ and Cl- ions. Once the K+ ions are dissolved, they quickly find negatively charged soil particles, and they will then temporarily stick to them until plant roots find and take up the K+ ions. While there are many other factors at work (presence of other ions in the soil, soil pH, etc.), this is the basis for how plant nutrient uptake works.
pH of soil: Optimum is 6
The pH value of soil is one of many environmental conditions that affect the quality of plant growth and nutrient availability, so it is an important factor to consider when looking at a soil analysis report, but surprisingly, it isn’t the MOST important.
pH is the measure of the acidity and alkalinity found within the soil, with a scale ranging from 0 to 14, with 7 considered neutral. Numbers less than 7 indicate acidity, while numbers greater than 7 indicate alkalinity, according to Clemson University. In reference to turfgrass, Houston area grasses such as St Augustine, Bermuda, and Zoysia thrive at a pH of around 6.
Of course, different plants thrive best in different soil pH ranges. For example, azaleas, rhododendrons, blueberries, and conifers thrive best in acid soils (pH 5.0 to 5.5). Vegetables, grasses, and most ornamentals do best in slightly acidic soils (pH 5.8 to 6.5). Soil pH values above or below these ranges for these plants may result in less vigorous growth and nutrient deficiencies.

Nutrients for healthy plant growth are divided into primary, secondary, and micronutrients. Nitrogen (N), phosphorus (P), and potassium (K) are primary nutrients that are needed in relatively large quantities compared to other plant nutrients. Calcium (Ca), magnesium (Mg), and sulfur (S) are secondary nutrients that are required by the plant in lesser quantities but are no less essential for healthy plant growth than the primary nutrients. Zinc (Zn) and manganese (Mn) are micronutrients that the plant (in very small amounts) requires. Most secondary and micronutrient deficiencies are easily corrected by keeping the soil at the optimum pH value.
Conclusion on pH
The major impact that extremes in pH have on plant growth is related to the availability of plant nutrients or the soil’s concentration of plant-toxic minerals. At pH values of 6.5 and above, phosphorus and most micronutrients become less available. In highly acid soils, aluminum and manganese can become more available, causing toxicity to the plant. Phosphorus, magnesium, and calcium are less available to plants with low pH values.
Organic Matter Percent: Optimum is 3-5

According to the USDA, soil organic matter (SOM) is the organic component of soil, consisting of three primary parts, small (fresh) plant residues and small living soil organisms, decomposing (active) organic matter, and stable organic matter (humus). Soil organic matter serves as a reservoir of nutrients for crops, provides soil aggregation, increases nutrient exchange, retains moisture, reduces compaction, reduces surface crusting, and increases water infiltration into the soil.
Anions in Soil
Sulfur: Optimum is 25-50
Sulfur is an anion, so, in the soil solution, it is very leachable, according to Soil Doctor. The levels of sulfur indicated on a soil test indicate how much precipitation or water runs through the soil profile. So If sulfur shows as low on a soil test, it’s getting leached out. In those types of soils, the majority of sulfur is actually supplied through organic matter. 90% of sulfur in soil is found in organic matter. So as biology naturally mineralizes the organic matter, it releases sulfur for plants. While plants take up about as much sulfur as phosphate, much more leaches yearly than phosphorus.
Mehlich III Phosphorous: Optimum is 250-500
Phosphorus, or “P,” is a primary plant nutrient that, in the metabolic processes, is responsible for energy transfer throughout the plant. It is crucial to introduce phosphorus when first establishing turf grass, which remains important as the grass continues to grow. The presence of phosphorus in the soil helps turf grow lush and thick because it promotes strong root growth. A soil test showing an abundance of phosphorus will tell you the plant will grow more efficiently, says Joe over at Turf Care Supply.
How Phosphorous Benefits Your Lawn: Enhancing Plant Energy Reactions; phosphorus plays a vital role in the energy transfer of plants. Phosphorus is responsible for the source of energy that drives many chemical reactions within plants: ADP (adenosine diphosphate) and ATP (Adenosine triphosphate), setting the stage for many chemical processes essential to plant health to occur.
Assisting in the Transfer of Genetic Material from One Generation to the Next, this primary nutrient is a vital component of the building blocks of DNA. The building blocks of genes and chromosomes that are transferred from One Generation to the next essentially provide the “blueprint” for plant growth and development.
Optimizing the Transfer of Sugars and Starches within Plants; photosynthesis is the most important chemical reaction for plants. Photosynthesis is the reaction that utilizes the sunlight’s energy to convert carbon dioxide and water, in the presence of chlorophyll, into simple sugars and future energy sources. The sugars are used as future building blocks for the cell structure and storage components. Energy is then stored in the form of Adenosine Triphosphate (ATP), rendering it available as an energy source for other reactions in the plant.
Exchangeable Cations in Soil
Calcium
The University of Florida says that Ca, or Calcium, is absorbed by turfgrass as Ca2+ (a positively-charged divalent cation), and, under normal conditions, turfgrass receives sufficient quantities of Ca2+ from the soil without the need to apply Ca fertilizers. It is also required for cell membranes to function properly and likely serves to bind phospholipids or membrane proteins. Most Ca in turfgrasses is found in cell vacuoles or serves as a structural component of cell walls (Kinzel 1989). Note that calcium activates some enzymes but inhibits others (Salisbury and Ross 1992). Translocation of Ca into phloem tissues is limited, so deficiency symptoms, although exceedingly rare, will appear on newer leaves/tissues first. Deficiency may appear as twisted or deformed tissue in roots, stems, or leaves where cell division occurs. Leaf blades may appear rose to brown in color, and the lead tips and/or margins may wither (Carrow et al. 2001).
Magnesium
Soluble magnesium (Mg) exists in soils primarily as Mg2+ (positively charged divalent cation) and is an essential element for all plants. Because Mg is often applied to turfgrasses in both granular and foliar forms, it is necessary to understand the function of Mg in the plant, the dynamics of Mg in the soil, and the forms of Mg fertilizers. Additionally, it is also necessary to be aware of the most current research regarding turfgrass responses to Mg in Texas so that you may make a more informed decision regarding Mg applications to turf grass.
The primary function of Mg in turfgrass is to serve as the central atom in the chlorophyll molecule. As much as 25% of plant Mg is used for this purpose (Carrow et al. 2001). Other functions of Mg in turfgrasses include enzyme activation, stabilization of ribosomes during protein synthesis, and maximization of energy during phosphate transfer (Havlin et al. 1999). Chlorophyll provides the green color in leaves, so a lack of Mg leads to a lack of green color (“chlorosis”), and turf can look pale and “hungry” for fertilizer even if other nutrients, such as nitrogen (N) and phosphorus (P), are adequate. Magnesium is mobile within the plant; thus, deficiency symptoms typically appear as chlorosis on older leaves first.
Potassium
Potassium is important in turf grass. The folks over at Jonathan Green say that nutrients supplied by soil and nature alone are frequently insufficient for healthy grass growth. Potassium or potash, as it is sometimes called, is the third number listed on fertilizer bags, such as 10-6-4. Potassium’s chemical symbol is “K,” and it is a macronutrient like nitrogen, and phosphorous, which means they are needed in larger (macro) amounts for healthy plant growth than micronutrients which are needed in smaller (micro) amounts such as iron, magnesium, and zinc. Many lawn fertilizers emphasize the first number, nitrogen because it helps grow green, lush grass, but potassium also plays a critical role in plant growth and health.
Potassium assists in better water and nutrient uptake while helping synthesize proteins and starches. Potassium also helps the grass build thicker cell walls and stay healthy. It also strengthens the plant so it can withstand various stresses such as drought, heat, cold, and disease. An application of potassium in the spring months can contribute to the grasses’ ability to withstand summer stresses better.
Sodium

Soil is composed of tiny particles bound together, creating larger particles, which is what makes up the “soil structure.” This binding of soil particles allows water and air to filter and collect in the soil. Soils with a higher percentage of clay (loamy) will hold more water and nutrients. Sandy soil does not have the same structure or holding capacity, so water and nutrients will leach quickly. A soil test will show that saline soils have a relatively high number of sodium ions compared to healthy soils.
Excessive salt destroys the soil structure, attracts water, and blocks its absorption to plant roots. As a result, plants may exhibit signs of drought even when the soil is wet or waterlogged. Other signs of salt damage may be water pooling on the surface without penetrating. Just as the results of high soil salinity on plants is seen in leaf and stem burns, it is hard on earthworms and microorganisms as well, according to Lawn Care Academy.
Saline Soils: This is Soil that contains high total soluble salts that can adversely affect plant health. An important note is that saline soils still have good soil structure intact. Saline soils must have chlorine present (Cl), whereas sodic soils usually lack chlorine. Soil pH for saline soils is usually between 7 and 8.5. An older name for saline soil is “white alkali” since a white salt film is often present on dry patches of bare ground.
Sodic Soils: Sodic Soil has high enough levels of sodium ions to affect soil structure. This means the sodium binds to clay particles, so the clay in the soil does not stick together when the soil becomes moist. It often becomes easily waterlogged, not allowing water to drain. When it does dry out, it becomes like concrete and is difficult for plant roots to penetrate. Because the soil does not hold together, the soil easily erodes away, especially on hillsides. The plants exhibit drought stress even with what appears to be adequate moisture. The pH in sodic soil is usually between 8.5 and 10.

Right: Soil with poor and dense structure (sodic soil).
The terms covered here are our most significant concerns and what we feel is the most important on a soil test report. The Base Saturation and Trace Elements are certainly part of a healthy turf conversation but are easily addressed and corrected with a carbon-rich program like the one we have here at GreenGate Turf.
SOURCES:
https://turfcaresupply.com/joe-knows-cation-exchange-capacity
hgic.clemson.edu/factsheet/changing-the-ph-of-your-soil
https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_053264.pdf
https://www.soildoctorconsulting.com/pro-tips/2020/4/27/sulfur
https://www.turfcaresupply.com/phosphorus-role-in-lawn-health
https://edis.ifas.ufl.edu/publication/EP554
https://edis.ifas.ufl.edu/publication/EP555
https://www.jonathangreen.com/blog/potassium-is-important-in-turf-grass.html
https://www.lawn-care-academy.com/soil-salinity.html
Havlin, J.L., J.D. Beaton, S.L. Tisdale and W.L. Nelson. 1999. Soil fertility and fertilizers: an introduction to nutrient management. 6th ed. Upper Saddle River, New Jersey: Prentice Hall.
Carrow, R.N., D.V. Waddington and P.E. Rieke. 2001. Turfgrass soil fertility and chemical problems: assessment and management. Chelsea, Michigan: Ann Arbor Press.
Kinzel, H. 1989. “Calcium in the vacuoles and cell walls of plant tissue.” Flora 182: 99‒125.
Salisbury, F.B. and C.W. Ross. 1992. Plant physiology. 4th ed. Belmont, California: Wadsworth Pub. Co.
Images:
https://www.technologynetworks.com/analysis/articles/cation-vs-anion-definition-chart-and-the-periodic-table-322863
https://extension.uga.edu/publications/detail.html?number=C1019&title=Soil%20Salinity%20Testing,%20Data%20Interpretation%20and%20Recommendations